
ADHD stimulants in children and the side effects
What side effects should I be watching for if my child is taking stimulants
The American Academy of Pediatrics states that stimulant medications for children with ADHD are generally safe, but long-term use is associated with several potential adverse effects that require monitoring.[1] ADHD stimulants in children and the side effects
Growth: The most robust evidence, including the MTA study, shows that stimulants can cause a persistent reduction in growth velocity, typically resulting in a decrease of 1–2 cm from predicted adult height. This effect is most pronounced with higher and more consistent dosing, and while the impact lessens after three years, there is no evidence of compensatory rebound growth.[1]
Cardiovascular health: Stimulants cause mild, clinically insignificant increases in heart rate (1–2 bpm) and blood pressure (1–4 mm Hg). However, 5–15% of children may experience more substantial increases, so regular monitoring of vital signs is recommended. Although rare, sudden cardiac death has been reported, but the risk is not higher than in children not taking stimulants. The guideline recommends screening for personal and family history of cardiac symptoms or conditions (e.g., sudden death, Wolff-Parkinson-White syndrome, hypertrophic cardiomyopathy, long QT syndrome) before starting stimulants, with further evaluation if risk factors are present.[1]
Risk of substance use: The guideline does not report an increased risk of substance use disorders with stimulant treatment in children and adolescents.[1]
Other adverse effects: Common short-term effects include appetite loss, abdominal pain, headaches, and sleep disturbance. Rarely, stimulants may cause hallucinations or psychotic symptoms.[1]
American Academy of Pediatrics
Published October 2019
Building on the guideline summary, recent longitudinal studies and meta-analyses provide further nuance regarding the long-term safety profile of stimulant medications in children with ADHD.
Growth effects: Large-scale prospective studies and meta-analyses confirm that long-term stimulant use, particularly methylphenidate, is associated with modest reductions in height and weight velocity, most pronounced in the first 1–2 years of treatment. The ADDUCE study, a 2-year controlled trial, found no significant difference in height velocity between medicated and unmedicated children, though a transient reduction in weight velocity was observed at 6 months, with no persistent effect at 2 years. Meta-analyses suggest the average reduction in adult height is small (1–3 cm), and the clinical impact is generally minimal, especially when balanced against the risks of untreated ADHD. Some evidence indicates that limiting total lifetime dose or implementing drug holidays may mitigate these effects.[2-5]
Cardiovascular safety: Stimulants consistently cause small increases in heart rate and blood pressure (typically 2–5 bpm and 1–4 mm Hg, respectively). The ADDUCE study and other long-term observational data show these changes are generally not clinically significant, but regular monitoring is advised. Large registry studies and meta-analyses have not demonstrated an increased risk of serious cardiovascular events (e.g., sudden death, stroke, myocardial infarction) in children treated with stimulants, though a modest risk cannot be entirely excluded, especially in those with underlying cardiac conditions.[2][6-8]
Risk of substance use: Longitudinal research indicates that stimulant treatment in childhood does not increase—and may even reduce—the risk of later substance use disorders. Concerns about diversion and misuse are more relevant in adolescents and adults, but not in younger children.[5][8]
Other adverse effects: Psychiatric and neurological adverse events (e.g., tics, psychosis-like symptoms) are rare and not consistently associated with long-term stimulant use. The ADDUCE study found no increased risk of depression, suicidality, or neurological symptoms over two years of methylphenidate treatment.[2]
The following table from the NEJM review summarizes recommended management strategies for common adverse events during ADHD medication treatment, including growth and cardiovascular monitoring:
Table 3 provides practical guidance for monitoring and managing adverse effects, emphasizing regular measurement of height, weight, heart rate, and blood pressure, and considering dose adjustments or drug holidays if clinically significant changes occur.
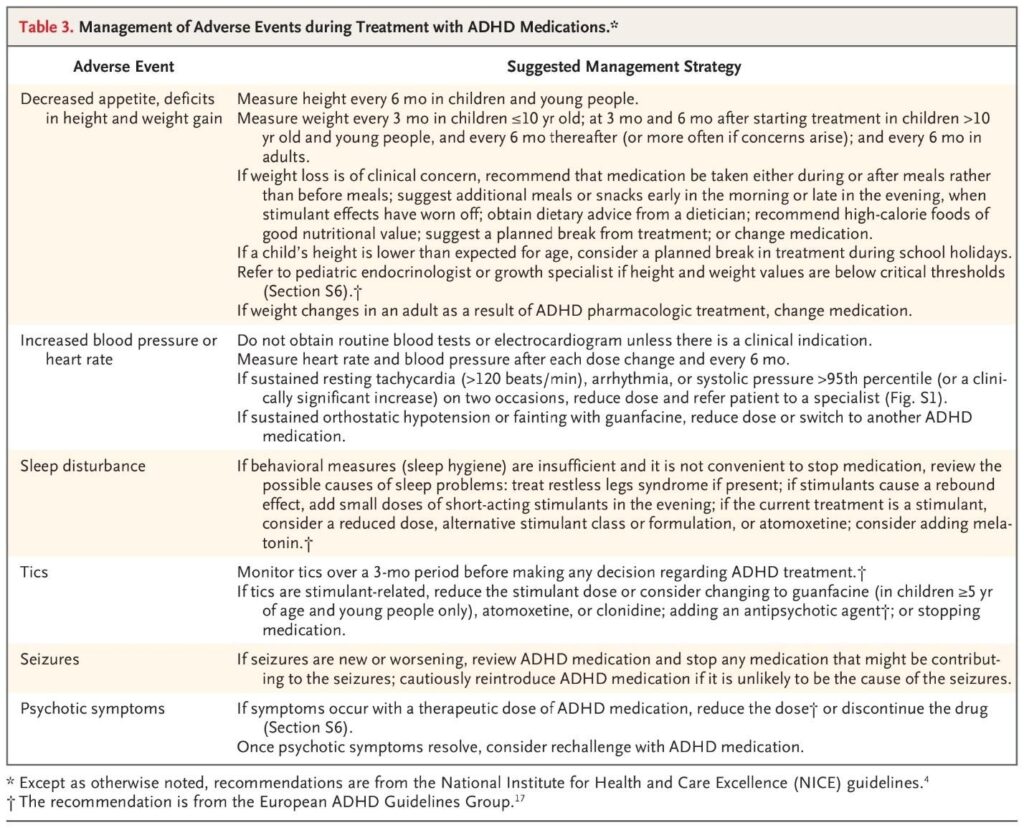
Table 3. Management of Adverse Events during Treatment with ADHD Medications.*
Pharmacologic Treatment of Attention Deficit–Hyperactivity Disorder. N Engl J Med. 9 September 2020.
Wolraich ML, Hagan JF, Allan C, et al.
Pediatrics. 2019;144(4):e20192528. doi:10.1542/peds.2019-2528.
Man KKC, Häge A, Banaschewski T, et al.
The Lancet. Psychiatry. 2023;10(5):323-333. doi:10.1016/S2215-0366(23)00042-1.
Carucci S, Balia C, Gagliano A, et al.
Neuroscience and Biobehavioral Reviews. 2021;120:509-525. doi:10.1016/j.neubiorev.2020.09.031.
Charach A. Journal of the American Academy of Child and Adolescent Psychiatry. 2020;59(8):929-930. doi:10.1016/j.jaac.2019.10.004.
Wilens T, Stone M, Spencer TJ.
Journal of the American Academy of Child and Adolescent Psychiatry. 2023;62(12):1305-1307. doi:10.1016/j.jaac.2023.05.018.
6. Attention-Deficit/Hyperactivity Disorder Medications and Long-Term Risk of Cardiovascular Diseases.
Zhang L, Li L, Andell P, et al.
JAMA Psychiatry. 2024;81(2):178-187. doi:10.1001/jamapsychiatry.2023.4294.
Cortese S.
The New England Journal of Medicine. 2020;383(11):1050-1056. doi:10.1056/NEJMra1917069.
Posner J, Polanczyk GV, Sonuga-Barke E.
Lancet (London, England). 2020;395(10222):450-462. doi:10.1016/S0140-6736(19)33004-1.




